When funders support groundbreaking life science research, they are investing in discoveries that could save lives. But what if that same research, in the wrong hands or under unsafe conditions, could cause harm? It’s a question every funder of infectious disease research should be asking—and now there’s a straightforward tool to help answer it.
The Challenge: Balancing Innovation with Safety and Security
Life science research moves quickly. From studying pandemic pathogens to developing new vaccines, the work is vital. But here’s the reality: research settings vary in terms of their ability to uphold safety standards, and not all research carries the same level or type of risk. Some studies involve pathogens with the potential to spread rapidly. Others might generate knowledge that could be misused by malicious actors.
For funders, the challenge is to identify these risks early—without slowing critical research.
The Solution: A Simple Framework to Assess Risk
NTI’s Guidance for Assessing Biosecurity and Biosafety Risks gives funders a practical framework to review research proposals. With three clear steps, funders can filter projects easily and identify potential risks.
Step 1: Rapid Screening – Filter Out the Low-Risk Stuff
The first step takes minutes, not weeks. It’s simply asking: Does this proposal involve high-risk work?
Specifically, it looks for research that involves:
- Pathogens known to cause epidemics or pandemics (like influenza viruses or Ebola)
- Modifications that could make a pathogen more dangerous (such as increasing its ability to spread or resist treatments)
- Biological agents with unknown risks that could potentially harm large populations
If the answer is ‘no’ across the board, funders are done with this assessment. Apply the standard proposal review process and move forward. This keeps low-risk research moving at full speed.
If the answer is yes to any of these, then funders dig deeper.
Step 2: Detailed Assessment – Understanding Real Risk
For higher-risk proposals, four critical questions must be answered before funding:
- Can this research be done safely and securely? Do they have the right facilities, trained staff, and safety protocols?
- Have they minimized the risks? Are there procedures in place to prevent accidents or theft?
- Are the public health benefits substantial and equitable? Will this research deliver real-world benefits that reach communities globally, not just wealthy nations?
- Is there a safer alternative? Could different methods achieve the same scientific goals with less risk?
The guidance provides specific questions to assess the magnitude of potential harm (Could this pathogen spread widely? Could the research make it more dangerous?) and the probability of harm (Are they using procedures that create infectious aerosols? What’s the facility’s safety track record?).
At this point, funders may identify that some research carries too much risk compared to potential benefits. These proposals should not proceed. However, this isn’t about saying no to important research—it’s about making informed decisions based on real risk factors.
Step 3: Risk Mitigation – Making Safe Research Safer
Even high-risk research that is worth pursuing can proceed safely with the right safeguards. The guidance walks funders through practical mitigation measures:
- Biosafety standards: Ensuring facilities meet WHO Laboratory Biosafety Manual standards and have qualified Biosafety Officers
- Biosecurity protections: From personnel screening to physical security to controlling access to dangerous materials
- Information security: Managing sensitive research findings before publication to prevent misuse
- Emergency preparedness: Having clear protocols if unexpected high-consequence findings emerge
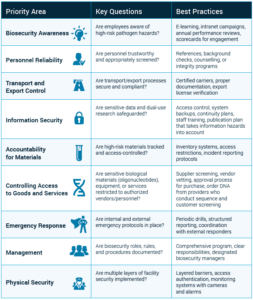
The guidance even includes a helpful table (Table 1) of biosecurity priority areas—covering everything from personnel reliability to transport controls to emergency response protocols.
Getting Started is Simple
The beauty of this guidance is its flexibility. Whether you’re a large government agency or a private foundation, you can adapt these steps to fit your review process. Start with the Rapid Screening for all proposals. Reserve detailed assessments for the proposals that truly need them. And use the risk mitigation section as a checklist when you’re ready to fund.
You don’t need to be a biosecurity expert to use this tool effectively—that’s the point. It translates complex safety concepts into clear, actionable questions. If you’re funding infectious disease research, this guidance helps you.
- Protect your investment and reputation by ensuring funded work meets safety standards
- Fulfill your responsibility to prevent both accidental infections and deliberate misuse
- Make faster, better-informed decisions without getting bogged down reviewing low-risk proposals
- Join a global community of responsible funders committed to safety and security
This guidance was developed to support the International Bio Funders’ Compact—a growing coalition of research funders and investors committed to integrating biosafety and biosecurity into their practices. By adopting this guidance, you’re not just protecting your own portfolio; you’re joining a global effort to make life science research safer while supporting innovation. Further, the Bio Funders Forum provides a space for funders to share best practices, learn from each other’s experiences, and collectively strengthen biosecurity across the research ecosystem.
The Bottom Line
Funding life science research means funding hope: hope for new treatments, better vaccines, and deeper understanding of the biological world. But this works best when it’s paired with responsibility.
This framework gives you a practical way to fund boldly without sacrificing safety and security. It’s not about creating barriers to research—it’s about building the right guardrails so that transformative research can proceed with confidence.
